Answer:
0.2 nm
Step-by-step explanation:
As we know that the energy of the nth level of one dimensional potential box is,
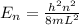
And also it is known that,
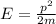
Put this in the above equation.
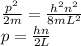
Now, de Broglie wavelength is,
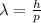
Therefore,
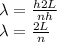
Given that, the width of 1 dimension box is,
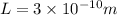
For the second excited state n=3,
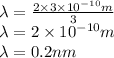
De Broglie wavelength of the particle is 0.2 nm.