Answer:
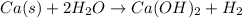
Step-by-step explanation:
Given:
In a balanced chemical equation, the number of atoms of each element are equal on both sides.
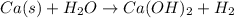
No. of atoms of Ca in left hand side = 1
No. of atoms of Ca in right hand side = 1
So, no. of atoms of Ca is balanced
Now,
No. of atoms of O in left hand side = 1
No. of atoms of O in right hand side = 2
Now multiply H2O with 2
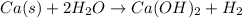
No. of atoms of O are balanced on each side
No. of atoms of H in left hand side = 4
No. of atoms of H in right hand side = 4
Now, each element is balanced
So coefficient of H2O after balancing = 2