Answer:
For A: The mass of oxygen per gram of sulfur for sulfur dioxide is 0.997 g
For B: The mass of oxygen per gram of sulfur for sulfur trioxide is 1.5 g
Step-by-step explanation:
The chemical equation for decomposition of sulfur dioxide follows:
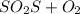
We are given:
Mass of sulfur = 3.47 g
Mass of oxygen = 3.46 g
To calculate the mass of oxygen per gram of sulfur, we apply unitary method:
For every 3.47 g of sulfur, 3.46 g of oxygen will form.
So, for 1 gram of sulfur,
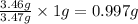 of oxygen will form.
of oxygen will form.
Hence, the mass of oxygen per gram of sulfur for sulfur dioxide is 0.997 g
The chemical equation for decomposition of sulfur trioxide follows:
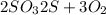
We are given:
Mass of sulfur = 5.50 g
Mass of oxygen = 8.25 g
To calculate the mass of oxygen per gram of sulfur, we apply unitary method:
For every 5.50 g of sulfur, 8.25 g of oxygen will form.
So, for 1 gram of sulfur,
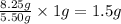 of oxygen will form.
of oxygen will form.
Hence, the mass of oxygen per gram of sulfur for sulfur trioxide is 1.5 g