Answer:
7.69x
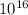
Step-by-step explanation:
The ionization is the energy necessary to transform an atom in an anion. So, it's the energy it requires to gain an electron.
This ionization occurs for the photoelectric effect - the emission of electrons by metal using photons. So, if the efficiency is 100%, 1 photon can ionize 1 atom, but for an effficiency of 13.0%:
N =
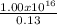
N = 7.69x
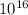
Where N is the number of photons.