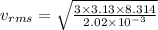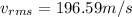Answer:
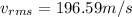
Given:
Temperature, T = 3.13 K
molar mass of molecular hydrogen, m = 2.02 g/mol =
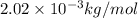
Solution:
To calculate the root mean squarer or rms speed of hydrogen molecule, we use the given formula:
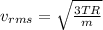
where
R = rydberg's constant = 8.314 J/mol-K
Putting the values in the above formula: