Answer: The mass of ammonia formed is 22.61 grams
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
Given mass of hydrogen gas = 4 g
Molar mass of hydrogen gas = 2 g/mol
Putting values in equation 1, we get:
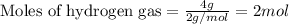
Given mass of nitrogen gas = 30 g
Molar mass of nitrogen gas = 28 g/mol
Putting values in equation 1, we get:
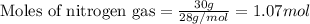
The chemical equation for the formation of ammonia follows:
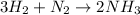
By Stoichiometry of the reaction:
3 moles of hydrogen gas reacts with 1 mole of nitrogen gas.
So, 2 moles of hydrogen gas will react with =
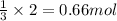 of nitrogen gas.
of nitrogen gas.
As, given amount of nitrogen gas is more than the required amount. So, it is considered as an excess reagent.
Thus, hydrogen gas is considered as a limiting reagent because it limits the formation of product.
By Stoichiometry of the reaction:
3 moles of hydrogen gas produces 2 moles of ammonia
So, 2 moles of hydrogen gas will produce =
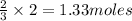 of ammonia
of ammonia
- Now, calculating the mass of ammonia from equation 1, we get:
Molar mass of ammonia = 17 g/mol
Moles of ammonia = 1.33 moles
Putting values in equation 1, we get:
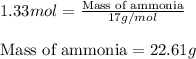
Hence, the mass of ammonia formed is 22.61 grams