Answer:
Concentration of KOH = 1.154 M
Step-by-step explanation:
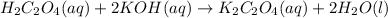
In the above reaction, 1 mole of oxalic acid reacts with 2 moles of KOH.
Mass of oxalic acid = 0.604 g
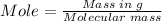
Molecular mass of oxalic acid = 90.03 g/mol
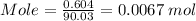
1 mol of oxalic acid reacts with 2 moles of KOH
0.0067 mol of oxalic acid reacts with
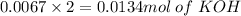
Volume of the solution = 27.02 mL = 0.0272 L
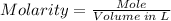
No. of mole of KOH = 0.0134 mol
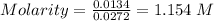
Concentration of KOH = 1.154 M