Answer:
326149.2 KJ
Step-by-step explanation:
The heat transfer toward and object that suffered an increase in temperature can be calculated using the expression:
Q = m*cv*ΔT
Where m is the mass of the object, cv is the specific heat capacity at constant volume, which basically means the amount of heat necessary for a 1kg of water to increase 1C degree in temperatur, and ΔT is the change in temperature.
A 65000 L swimming pool will have a mass of:
65000L *
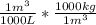 = 65000 kg
= 65000 kg
The specific heat capacity at constant volume of water is equal to 4.1814 KJ/KgC.
We replace the data and get:
Q = m*cv*ΔT = 65000 kg * 4.1814 KJ/KgC * 1.2°C = 326149.2 KJ