Answer: 0.67 g
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
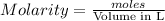
![moles of [tex]Na_2S_2O_3=Molarity* {\text {Volume in L}}=0.150* 0.0352=5.28* 10^(-3)moles](https://img.qammunity.org/2020/formulas/chemistry/college/ldpe6meq1i8474sqdsg057aiwrka2bmc0c.png)
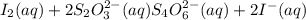
According to stoichiometry:
2 moles of
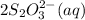 require 1 mole of
require 1 mole of
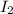
Thus
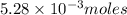 require=
require=
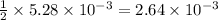 moles of
moles of
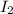
Mass of
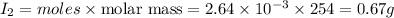
Thus 0.67 g of iodine are present in the solution.