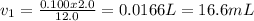Answer:
Sodium chloride solution:
First you need to calculate the mass of salt needed (done in the explanation), which is 58.44g. Then it have to be weighted in an analytical balance in a weighting boat and then transferred into a 2L volumetric flask that is going to be filled until the mark with distilled water.
Sulfuric acid dilution:
First you need to calculate the volume needed (done in the explanation), it is 16.6 mL. Using a graduated pipette one measures this volume and transfer it into a 2L volumetric flask that is already half filled with distilled water, and then one fills it until its mark.
Explanation:
Sodium chloride solution:
Each liter of a 0.500M solution has half mol, so 2L of said solution has 1 mol of salt. Sodium chloride molar mass is 58.44g/mol, so in 2L of solution there is 58.44g of salt. That`s the mass that`s going to be weighted and transferred to a 2L volumetric flask.
Sulfuric acid dilution:
This is the equation for dilution of solutions:
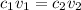
Where "c1" stands for the initial concentration (stock solution concentration), "v1" for the initial volume (volume of stock solution used), "c2" for the desired concentration and "v2" for the desired volume.
When we are diluting from a stock solution we want to know how much do we have to pipette from the stock solution into our volumetric flask. We do so by isolating the "v1" term from the dilution equation:
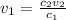
in this case that would be: