Step-by-step explanation:
The mass of electron,
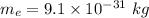
The mass of neutron,
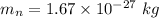
The De-Broglie wavelength is given by :
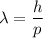
p is the momentum, p = m v
For electron,
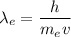
For neutron,
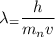
Since, an electron and a neutron have the same de Broglie wavelength. So,
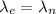
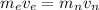
The mass of electron is less than the mass of neutron. So, the speed of electron is more than than the speed of neutron. As the kinetic energy is directly proportional to the mass. So, the correct option is (a) The electron has more kinetic energy and a higher speed.