Answer:
B) protons
Step-by-step explanation:
The total number of protons in an atom represents the atomic number of that element. An element is generally expressed as
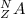
Where,
A is the element
N is the Mass number i.e., the number of neutrons and protons in an atom.
Z is the atomic number.
The elements are arranged according to their atomic number in the modern periodic table.