Answer:
The minimum amount is 6,9 g
Step-by-step explanation:
The extraction of benzoic acid with aqueous NaHCO₃ is an Acid-Base Reaction, where the benzoic acid loses a proton to the sodium bicarbonate, as follows:
(a) Ф-COOH + NaHCO₃ ↔ Ф-COO⁻ + NaH₂CO₃⁺
In order to calculate the amount of bicarbonate needed, some conversion factors need to be used:
10 g acid *
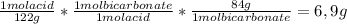
Thus, 6,9 g of sodium bicarbonate would need to be added in order to extract 10 g of benzoic acid