Answer:
0.8342 grams of ethylene glycol must be added to one liter of water.
Step-by-step explanation:
Volume of water or solvent = 1 L
Density of water = 1 g/L
Mass of the water = Density × Volume = 1 g/L × 1 L = 1 g
1 g = 0.001 kg
Molal freezing constant of water = 1.86 °C/m =1.86 °C/(mol/kg)
The van't Hoff factor contribution by ethylene glycol is 1 .
i = 1
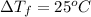
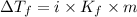
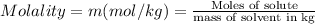
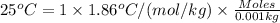
Moles of solute (ethylene glycol) = 0.013440860 moles
Mass of 0.013440860 moles of ethylene glycol:
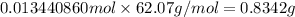
0.8342 grams of ethylene glycol must be added to one liter of water.