Answer:
0.4777 cm³
Step-by-step explanation:
Molar mass is defined as the mass of 1 mole of the substance.
The molar mass fo Ag is 107.8682 g/mol
Since, 1 mole of Ag contains 6.022×10²³ molecules of Ag
So,
6.022×10²³ molecules of Ag has a mass of 107.8682 g
Also,
1 molecule of Ag has a mass of 107.8682/6.022×10²³ g
2.8×10²² molecules of Ag has a mass of (107.8682/6.022×10²³)×2.8×10²² g
Mass of 2.8×10²² molecules = 5.0155 g
Given that density = 10.5 g/cm³
Volume = ?
So, volume:
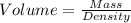
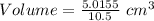
Volume = 0.4777 cm³