Answer:
Molar mass of the gas = 15.15 g/mol
Step-by-step explanation:
PV = nRT
Where,
P = pressure
n = No. of moles
R = Gas constant
T = Temperature
P = 698 torr, 1 torr = 0.00131579 atm
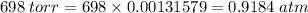
Temperature = 111 °C = 100 + 273.15 = 384.15 K
V = 48.7 L
R = 0.082057 L atm/mol K
Now, PV = nRT
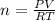
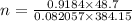
=1.4189 mol
Molar mass = Mass/ No. of moles
= 21.5/1.4189
=15.15 g/mol