Step-by-step explanation:
The given data is as follows.
m = 19.2 g, I = 15 A
The given reaction equation will be as follows.
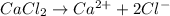
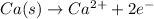
As, 1 mole will give
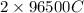 of charge. So, calculate the amount of charge deposited by 19.2 g as follows.
of charge. So, calculate the amount of charge deposited by 19.2 g as follows.
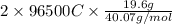
= 94404.791 C
Hence, 94404.791 C are needed to produce 19.2 g of Ca metal.
Therefore for 15 A, minutes required to produce 94404.791 C and 19.2 g of Ca(s) will be calculated as follows.
=
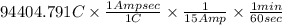
= 104.9 mins
Thus, we can conclude that 104.9 minutes are required if a cell runs at 15 A, how many minutes will it take to produce 19.2 g of Ca(s).