Answer:
engine B is more efficient.
Step-by-step explanation:
We know that Carnot cycle is an ideal cycle for all working heat engine.In Carnot cycle there are four processes in which two are constant temperature processes and others two are isentropic process.
We also kn ow that the efficiency of Carnot cycle given as follows
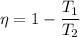
Here temperature should be in Kelvin.
For engine A
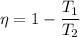
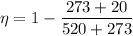
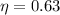
For engine B
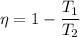
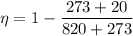
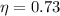
So from above we can say that engine B is more efficient.