Answer:
The age of the alloy is 73.94 years.
Step-by-step explanation:
For metal A:
Initial amount of metal A = x
Final amount after time t = 0.53 kg
Half life of metal A =
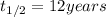
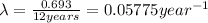
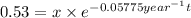 ..(1)
..(1)
For metal B:
Initial amount of metal B = x
Final amount after time t = 2.20 kg
Half life of metal B =
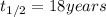
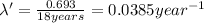
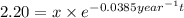 ..(2)
..(2)
Dividing (1) by (2)
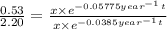
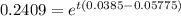
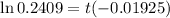
t = 73.94 years