Answer:
The atomic mass of iodine-131 is 130.9056485 amu.
Step-by-step explanation:
Beta-decay is the process in which a neutron gets converted into a proton and an electron releasing a beta-particle. The beta particle released carries a charge of -1 units.
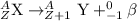
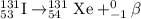
Energy released during beta particle emission = E

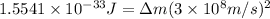
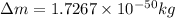
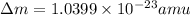
Mass of reactant = Mass of product + Δm
= Mass of beta particle + nuclide + Δm
Mass Beta-particle =
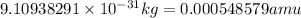
Mass of reactant =
=130.9051+0.000548579 amu +
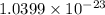 amu
amu
= 130.9056485 amu
The atomic mass of iodine-131 is 130.9056485 amu.