Answer: e) 9.43
Explanation: A buffer solution is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid and the pH of the buffer is calculated using Handerson equation:
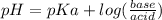
There are 25 mL of 0.50 M
 which is a weak base. 25 mL of 0.20 M HCl, a strong acid are added. Ammonia reacts with HCl to form its conjugate acid, ammonium ion. The net ionic equation will be:
which is a weak base. 25 mL of 0.20 M HCl, a strong acid are added. Ammonia reacts with HCl to form its conjugate acid, ammonium ion. The net ionic equation will be:
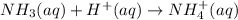
From above reaction, ammonia and HCl react in 1:1 mol ratio. Let's calculate the moles of each we have before the reaction. There is also, 1:1 mol ratio between HCl and ammonium ion.
moles of ammonia =
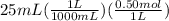
= 0.0125 mol
moles of HCl =
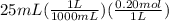
= 0.005 mol
Excess moles of ammonia = 0.0125 - 0.005 = 0.0075 mol
moles of ammonium ion formed = 0.005 mol
Total volume of the solution = 0.025 mL + 0.025 mL = 0.050 L
concentration of ammonia in buffer =
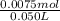
= 0.15 M
concentration of ammonium ion in buffer =
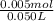
= 0.10 M
pKa is calculated from given Ka as:
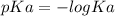
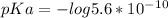
pKa = 9.25
Plug in the values in Handerson equation:
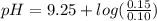
pH = 9.25 + 0.18
pH = 9.43
So, the correct choice is e) 9.43 .