Step-by-step explanation:
It is given that volume of
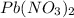 is 300 mL and molarity is 0.200 M.
is 300 mL and molarity is 0.200 M.
Volume of NaCl is 200 mL and molarity is 0.050 M.
The chemical reaction will be as follows.
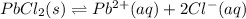
 for
for
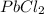 is given as
is given as
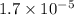 .
.
As, molarity is number of moles present in liter of solution.
Hence, moles of
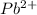 (aq) will be calculated as follows.
(aq) will be calculated as follows.
moles of
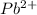 (aq) =
(aq) =
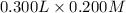
= 0.06 mol
![[Pb^(2+)]](https://img.qammunity.org/2020/formulas/chemistry/college/b4acgmyvdeowtue90jvnyrf5e2r0w481qd.png) =
=
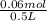
= 0.120 M
Mole of
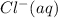 =
=
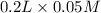
= 0.010 M
Now, Q =
![[Pb^(2+)][Cl^(-)]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/5bn8mod0blkzgj02s2q372sgq67b7rgc9c.png)
=
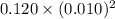
=
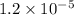
As, Q <
 hence, there will be no formation of
hence, there will be no formation of
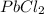 precipitate.
precipitate.