Step-by-step explanation:
Molar mass of potassium nitrate will be calculated as follows.
Molar mass
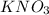 = molar mass of K + molar mass of N + 3 × molar mass of O
= molar mass of K + molar mass of N + 3 × molar mass of O
= 39.098 g/mol + 14.006 g/mol + 3 × 15.999 g/mol
= 102.102 g/mol
Now, adding the given amount of potassium nitrate present in each beaker as follows.
(2.3 + 1.91 + 5.985 + 0.52) g
= 10.715 g
Therefore, calculate number of moles as follows.
No. of moles =
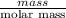
=
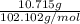
= 0.105 mol
Thus, we can conclude that 0.105 moles of potassium nitrate were recovered after the water evaporated.