Answer:
Approximately 50.67 mL of a 0.225 M NH₃ solution will react with 30.0 mL of a 0.190 M H₂SO₄ solution.
Step-by-step explanation:
Considering the balanced equation below, we have that 2 mol of NH₃ reacts with 1 mol of H₂SO₄:
2NH₃(aq) + H₂SO₄(aq) → (NH₄)₂SO₄(aq)
Therefore, we need to know the amount of H₂SO₄ present in the 30.0 mL given. We can use the following equation to calculate it:
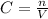
C is the concentration, n the number of moles of the solute and V is the volume.
H₂SO₄ :
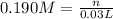 ∴ n = 5.7 x 10⁻³ mol
∴ n = 5.7 x 10⁻³ mol
The amount of NH₃ needed to react will be twice the amount of H₂SO₄, therefore:
NH₃:
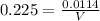 ∴ V = 50.67 mL
∴ V = 50.67 mL