Answer:
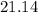 °C
°C
Step-by-step explanation:
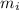 = mass of ice cube = 0.0600 kg
= mass of ice cube = 0.0600 kg
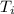 = initial temperature of ice cube = - 30.0°C
= initial temperature of ice cube = - 30.0°C
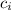 = specific heat of ice = 2030 Jkg⁻¹ C⁻¹
= specific heat of ice = 2030 Jkg⁻¹ C⁻¹
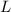 = Latent heat of fusion of ice to water = 334000 J/kg
= Latent heat of fusion of ice to water = 334000 J/kg
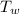 = initial temperature of water = 35.0°C
= initial temperature of water = 35.0°C
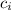 = specific heat of water = 4186 Jkg⁻¹ C⁻¹
= specific heat of water = 4186 Jkg⁻¹ C⁻¹
 = Final equilibrium temperature = ?
= Final equilibrium temperature = ?
Using conservation of heat
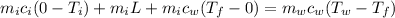
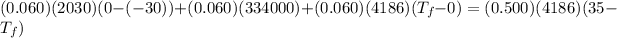
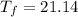 °C
°C