Answer:
The correct answer is option A.
Step-by-step explanation:
The expression of Gibbs's fee energy is given as:
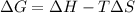
 = Change in Gibbs free energy
= Change in Gibbs free energy
 = Change in an entropy
= Change in an entropy
 = Enthalpy of reaction
= Enthalpy of reaction
T = Temperature at which reaction is going on.
We have:
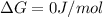
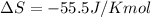
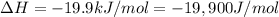
T =?
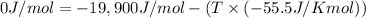
T = 359 K