Answer:
44,901 kilo Joule heat is released when
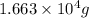 grams of ammonia is produced.
grams of ammonia is produced.
Step-by-step explanation:
Moles of ammonia gas produced :
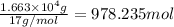
According to reaction, when 2 moles of ammonia are produced 9.18 kilo joules of energy is also released.
So, When 978.235 moles of ammonia gas is produced the energy released will be:
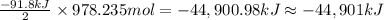
(negative sign indicates that energy is released as heat)
44,901 kilo Joule heat is released when
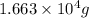 grams of ammonia is produced.
grams of ammonia is produced.