Answer: A. Liquefied
 boils at a very low temperature (–253.7°C).
boils at a very low temperature (–253.7°C).
B.
 gas does not conduct electricity.
gas does not conduct electricity.
C. At room temperature, its density is less than that of any other gas.
Step-by-step explanation:
Chemical property is defined as the property of a substance which is observed during a reaction where the chemical composition identity of the substance gets changed.
Example:
 reacts vigorously with oxygen
reacts vigorously with oxygen
 to form water.
to form water.
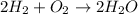
Physical property is defined as the property which can be measured and whose value describes the state of physical system. For Example: State, density etc.
Example: Liquefied
 boils at a very low temperature (–253.7°C).It only changes state.
boils at a very low temperature (–253.7°C).It only changes state.
 gas does not conduct electricity.It is only about movement of electrons.
gas does not conduct electricity.It is only about movement of electrons.
At room temperature,
 density is less than that of any other gas. It is only about mass present per unit volume.
density is less than that of any other gas. It is only about mass present per unit volume.