Answer:
option (c) is correct
Step-by-step explanation:
Half life of a substance is the time in which the element becomes half of is initial value.
half life, T = 8 days
Amount remaining, N = 10 % of original value
Let the original value is No.
N = 10% of No
N = 0.1 No
Let the time taken is t and the decay constant is λ.
The relation between the decay constant and the half life is given by
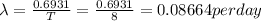
Us the equation of radioactivity
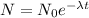
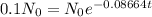
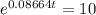
Taking natural log on both the sides, we get
0.08664 t = 2.303
t = 26.6 days