Step-by-step explanation:
It is given that,
Length of one dimensional box,
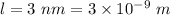
Wavelength of electron,
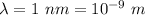
(a) We need to find the quantum number of this electron. The energy of electron in one dimensional box is given by :
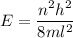
Also, energy is given by,
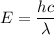
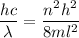
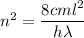
c is the speed of light
m is the mass of electron
h is the Planck's constant
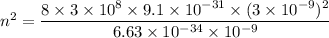
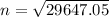
n = 172.18
or
n = 172
(b) For ground state energy, n = 1
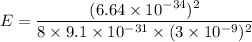
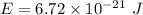
(c) We need to find the wavelength of a photon that is emitted in a transition from the energy level in part a to the first excited state (n=2). So,
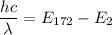
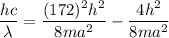
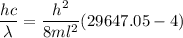
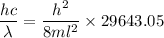
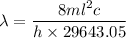
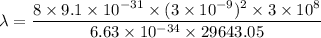
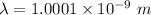
Hence, this is the required solution.