Answer:
Chemicals consumed: gasoline, oxygen and nitrogen.
Chemicals produced: carbon monoxide, carbon dioxide, water, sulfur oxides, nitrogen oxides, unburned gasoline or fuel and soot.
Step-by-step explanation:
Hello,
It is widely known that automobiles motion is caused by the gasoline combustion via an applied electric spark leveraging the flammability of the gasoline and the presence of oxygen into the engine. In such a way, we can exemplify such combustion process via the following widespread chemical reaction:
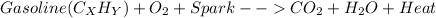
Whereas the formula of gasoline depends on the average composition of hydrocarbons composing it, typically octane; therefore, if we talk about the chemicals that are consumed and produced during the motion of a car, we ideally set gasoline and oxygen as the mainly consumed reactants and carbon dioxide and heat as the mainly produced species. Nonetheless, gasoline has traces of both sulfur and nitrogen as it comes from the crude oil distillation, in this manner, sulfur and nitrogen oxides could be produced as well during the combustion. In addition, complete combustion seldom happens as not all the oxygen consumes the entire fuel to completion, instead of that, incomplete combustion which produces carbon monoxide, unburned fuel and soot as well. Finally, as the engine uses air as the source of oxygen, it also contains nitrogen which is likely to react with oxygen to produce nitrogen oxides too.
Best regards.