Answer: True
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.
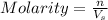
where,
n= moles of solute

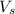 = volume of solution in L
= volume of solution in L
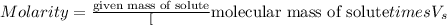
Thus when making a molar liquid solution from a dry chemical stock you must know the molecular weight of the compound.