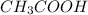Step-by-step explanation:
More is the electronegativity of atoms attached to the alpha carbon atom more will be the attraction of electron towards it. As a result, release of hydrogen ion will be easier.
Therefore, there will be increased in acidity of the compound.
Since, fluorine, chlorine and bromine are all group 17 elements and fluorine is more reactive than chlorine. On the other hand, chlorine is more reactive than bromine.
Hence, ability to attract the electrons towards itself will be maximum in fluorine and least in bromine.
Therefore, decreasing order of acidity of the given compounds is as follows.
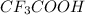 >
>
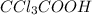 >
>
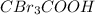 >
>