Answer:
Heat is absorbed by the system then the sign of Q is positive.
The system does work on the surroundings, then the sign of W is negative.
Step-by-step explanation:
Given that,
Absorbs heat = 174 J
Surrounding energy = 312 J
We need to calculate the heat
Using thermodynamics first law
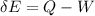
Where,
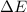 = Change in internal energy
= Change in internal energy
Q = heat change of the system
W = work
According to thermodynamics first law,
Heat is absorbed by the system then the sign of Q is positive.
The system does work on the surroundings, then the sign of W is negative.
Hence, This is the required solution.