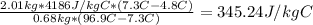Answer:
345.24 J/kgC
Step-by-step explanation:
The heat that Brass had to expell to drop its temperature from 96.9◦C to 7.3◦C is the same heat that the Water had to absorb to raise its temperature from 4.8◦C to 7.3◦C:
QBrass = Qwater
mb*cb*ΔTb = mw * cw * ΔTw
Where mb is the mass of brass, cb is the specific heat capacity of brass, ΔTb is the change in temperature that the brass suffered, mw is the mass of the water, cw is the specific heat of water and ΔTw is the change in temperature that the water suffered.
cb =
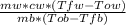
cb =