Answer : The mass in grams of a single formula unit of silver chloride will be

Step-by-step explanation:
Formula units : It is defined as lowest whole number ratio of ions in an ionic compound. It is calculate by multiplying the number of moles by Avogadro's number which is
 .
.
Single formula unit of silver chloride means that 1 mole of silver chloride.
We are given :
Number of moles of silver chloride = 1 moles
The molar mass of silver chloride = 143.32 g/mole
That means, 1 mole silver chloride contains 143.32 grams of silver chloride.
Now we have to determine the mass in grams of a single formula unit of silver chloride.
As,
 formula unit has mass = 143.32 g
formula unit has mass = 143.32 g
So, 1 formula unit has mass =
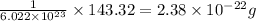
Therefore, the mass in grams of a single formula unit of silver chloride will be
