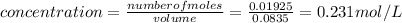Answer:
0.231 mol/L
Step-by-step explanation:
The first step is to write the balanced equation for this reaction:

The second step is to find the number of moles in the acid:
number of moles = volume * concentration
= 0.035 L * 0.275 mol/L
= 0.009625 mol
The third step is to use the molar ratio from the balanced chemical equation to find the number of moles of NaOH that can neutralize 0.009625 mol of sulphuric acid.
n(sulphuric acid) : n(sodium hydroxide)
1 : 2
0.009625 mol : x
x = 0.01925 mol
Fourth step is to calculate the concentration of sodium hydroxide: