Answer: The total number of electrons that must be shared between carbon and oxygen atoms are 8.
Step-by-step explanation:
A covalent compound is formed when sharing of electrons takes place between the atoms forming a compound.
Carbon is the 6th element of the periodic table having electronic configuration of
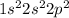
This element requires 4 electrons to complete its shell.
Oxygen is the 8th element of the periodic table having electronic configuration of
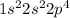
This element requires 2 electrons to complete its shell.
So, in order to complete the octet of both the elements, 2 oxygen atoms are required for 1 carbon atom.
Hence, the total number of electrons that must be shared between carbon and oxygen atoms are 8.