Step-by-step explanation:
The given diagram shows a water (
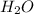 ) molecule. As atomic number of hydrogen is 1 so, its electronic distribution is 1 only. Also, atomic mass of a hydrogen atom is 1.
) molecule. As atomic number of hydrogen is 1 so, its electronic distribution is 1 only. Also, atomic mass of a hydrogen atom is 1.
This means it contains 1 proton and 0 neutron. And, in order to acquire stability a hydrogen atoms needs to gain only 1 electron.
Whereas atomic number of an oxygen atom is 8 and its electronic distribution is 2, 6. Also, atomic mass of oxygen is 16. It means that an oxygen atom contains 8 protons and 8 neutrons.
When n = 1 orbit then it means the number of electrons present in very first orbit of an atom.
When there will be sharing of electrons between the two chemically combining atoms then there will be formation of a covalent bond.
Therefore, according to the diagram:
- Each hydrogen atom contains 1 proton.
- Each oxygen atom contains 8 neutrons.
- 1 electron is needed to complete hydrogen's outer shell.
- 2 electron pairs are being shared between the hydrogen atoms and the bonded oxygen.
- Singe bond exists between the hydrogen and the oxygen atom.
- There are 6 electrons are in oxygen's outer orbit.
- The inner/outer orbit of oxygen has two electrons? It is true that only the inner orbital of oxygen atom contains 2 electrons. Whereas the outer orbital of a neutral oxygen atoms contains 6 electrons.