Answer:
5.104%
Explanation:
Given:
The accepted value for the mass density = 4.80 g/cc
Observed values:
5.13 g/cc
4.98 g/cc
5.06 g/cc
5.01 g/cc
Total number of observed values = 4
Mean of the observed values = ( 5.13 + 4.98 + 5.06 + 5.01 ) / 4 = 5.045 g/cc
Now,
The percentage error is calculated as:
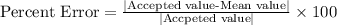 %
%
on substituting the values, we get
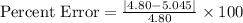 %
%
or
Percentage error = 5.104%