Answer : The molarity of chloride anion in the solution is 0.003318 mole/L
Explanation : Given,
Mass of
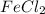 = 0.701 g
= 0.701 g
Volume of solution = 300 ml = 0.3 L
Molarity of AgCl = 14.0 M = 14.0 M
Molar mass of
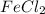 = 126.751 g/mole
= 126.751 g/mole
First we have to calculate the moles of
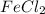 .
.
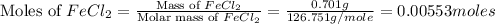
Now we have to calculate the moles of
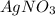 .
.
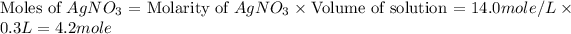
Now we have to calculate the limiting and excess reagent.
The balanced chemical reaction is,
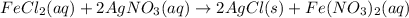
From the balanced reaction we conclude that
As, 1 moles of
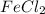 react with 2 mole of
react with 2 mole of
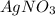
So, 0.00553 moles of
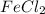 react with
react with
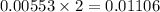 moles of
moles of
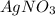
From this we conclude that,
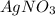 is an excess reagent because the given moles are greater than the required moles and
is an excess reagent because the given moles are greater than the required moles and
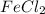 is a limiting reagent and it limits the formation of product.
is a limiting reagent and it limits the formation of product.
Now we have to calculate the moles of
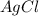 .
.
As, 1 moles of
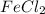 react to give 2 moles of
react to give 2 moles of
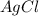
So, 0.00553 moles of
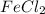 react to give
react to give
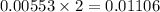 moles of
moles of
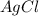
Now we have to calculate the molarity of
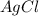 .
.
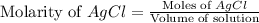
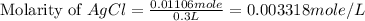
As we know that, 1 mole of AgCl in solution gives 1 mole of silver ion and 1 mole of chloride ion.
So, the molarity of chloride ion = Molarity of AgCl = 0.003318 mole/L
Therefore, the molarity of chloride anion in the solution is 0.003318 mole/L