Answer :
(a) The number of oxygen molecules present in 0.100 mL of gas is,
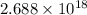
(b) The number of oxygen molecules present in 1.0 L of gas is,
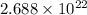
(c) The volume of oxygen gas is,
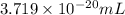
Explanation :
(a) To calculate the number of oxygen molecules present in 0.100 mL of gas.
As, 22400 mL of oxygen gas contains
 number of oxygen molecules
number of oxygen molecules
So, 0.100 mL of oxygen gas contains
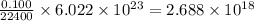 number of oxygen molecules
number of oxygen molecules
(b) To calculate the number of oxygen molecules present in 1.00 L or 1000 mL of gas.
As, 22400 mL of oxygen gas contains
 number of oxygen molecules
number of oxygen molecules
So, 1000 mL of oxygen gas contains
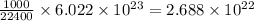 number of oxygen molecules
number of oxygen molecules
(c) To calculate the volume of oxygen gas for one molecule of oxygen.
As,
 number of oxygen molecules present in 22400 mL of oxygen gas
number of oxygen molecules present in 22400 mL of oxygen gas
So, 1 oxygen molecules present in
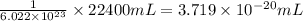 of oxygen gas
of oxygen gas