Answer: The change in entropy of the lead when 2.0 kg of molten lead at its melting point temperature solidifies is 20 cal/K.
Step-by-step explanation:
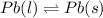
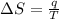
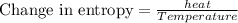
T is the temperature at which the process is carried out, here the temperature is constant, 327.5°C =
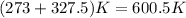 ,because only phase change occurs.
,because only phase change occurs.
q = heat given in the process =
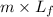
m = mass of lead = 2.0 kg
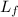 = latent heat of fusion of lead = 5.9 kcal/kg
= latent heat of fusion of lead = 5.9 kcal/kg
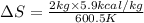
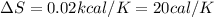
Thus the change in entropy of the lead when 2.0 kg of molten lead at its melting point temperature solidifies is 20 cal/K.