Answer:
3.3535 g
Step-by-step explanation:
Considering:

Or,
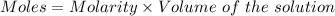
Given :
For iron(III) nitrate :
Molarity = 0.404 M
Volume = 52.0 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 52.0×10⁻³ L
Thus, moles of iron(III) nitrate :
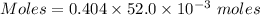
Moles of iron(III) nitrate = 0.021 moles
1 mole of iron(III) nitrate forms 1 mole of iron(III) hydroxide which further forms 1 mole of iron(III) oxide.
Thus, moles of iron(III) oxide formed from 0.021 moles of iron(III) nitrate = 0.021 moles
Also, molar mass of iron(III) oxide = 159.69 g/mol
So, mass of iron(III) oxide = 0.021 moles × 159.69 g/mol = 3.3535 g