Answer:
3.5434 eV
Step-by-step explanation:
For a particle with kinetic energy E and mass m , the wavelength associated is given by the following relation,
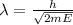
E =
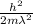
Putting the values we get
E =
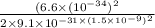
=1.063 x 10⁻²¹ J
= .0066 eV.
Energy of¹light in terms of eV
= 1244 / 350 = 3.55 eV.
Work function = 3.55 - 0.0066 = 3.5434 eV.