Answer:
pH = 11.95≈12
Step-by-step explanation:
Remember the reaction among aqueous acetic acid (
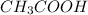 ) and aqueous sodium hydroxide (NaOH)
) and aqueous sodium hydroxide (NaOH)
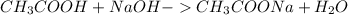
First step. Need to know how much moles of the substances are present
![0.1 (mol NaOH)/(L) * 0.030 L = 0.003 mol NaOH</p><p>[tex]0.1 (mol CH_3COOH)/(L) * 0.025 L= 0.0025 mol CH_3COOH</p><p></p><p>Second step. Know wich substance is in excess.</p><p>0.0025 mol CH_3COOH * [tex]1 mol NaOH/ 1 mol CH_3COOH]() = 0.0025 mol NaOH
= 0.0025 mol NaOH
0.003 mol NaOH *
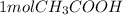 / 1 mol NaOH = 0.003 mol CH_3COOH[/tex]
/ 1 mol NaOH = 0.003 mol CH_3COOH[/tex]
NaOH is in excess. Now, how much?
0.003 mole NaOH - 0.0025 mole NaOH = 0.0005 mole NaOH
Then, that amount in excess would be responsable for the pH.
Third step. Know the pH
Remember that pH= -log[H+]
According to the dissociation of water equilibrium
Kw=[H+]*[OH-]= 10^(-14)
The dissociation of NaOH is
NaOH ->
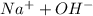
Now, concentration of OH^{-}[/tex] would be given for the excess of NaOH.
[OH-]= 0.0005 mole / 0.055 L = 0.00909 M
Careful: we have to use the total volumen
Les us to calculate pH
![pH= -log [H+]\\pH= -log (K_w)/([OH-]) \\pH= 11.95](https://img.qammunity.org/2020/formulas/chemistry/college/3uy15f82535xf6l56ycm0d5fprutlubhc8.png)