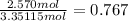Answer:
Step-by-step explanation:
Assume you have 100 g of the stack gas. Calculate the mass of each species in this sample according their percentages.
mass of sulphur : 6% of 100 g = 6 g
mass of oxygen : 22% of 100 g = 22 g
mass of nitrogen : 72% of 100 g = 72 g
Now calculate the number of moles of each species
number of moles of sulphur :
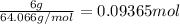
number of moles of oxygen:
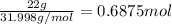
number of moles of nitrogen:
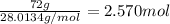
Now to calculate the mol fraction of each we use the formula:
mol fraction of A =
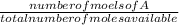
Therefore
mole fraction of sulphur =
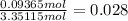
mole fraction of oxygen =
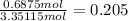
mole fraction of nitrogen =