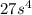Answer: The solubility product of
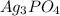 is
is
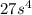
Step-by-step explanation:
Solubility product is defined as the equilibrium constant in which a solid ionic compound is dissolved to produce its ions in solution. It is represented as
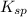
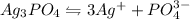
We are given:
Solubility of
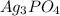 = S mol/L
= S mol/L
By stoichiometry of the reaction:
1 mole of
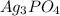 gives 3 moles of
gives 3 moles of
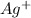 and 1 mole of
and 1 mole of
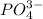 .
.
When the solubility of
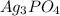 is S moles/liter, then the solubility of
is S moles/liter, then the solubility of
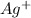 will be 3S moles\liter and solubility of
will be 3S moles\liter and solubility of
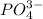 will be S moles/liter.
will be S moles/liter.
Expression for the equilibrium constant of
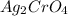 will be:
will be:
![K_(sp)=[Ag^+]^3[PO_4^(3-)]](https://img.qammunity.org/2020/formulas/chemistry/college/gq8l04kh1bjoxzpho9d88dizt9spgmcb5d.png)
![K_(sp)=[3s]^3[s]=27s^4](https://img.qammunity.org/2020/formulas/chemistry/college/u7sgivw0e4w39fv0jogujvlbhfvx99cs82.png)
The solubility product of
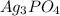 is
is