Answer: In this equation, the coefficient in front of the
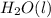 is 3.
is 3.
Explanation: Looking at the given reaction, Iron is oxidizing as its oxidation number is changing from +2 to +3. reduction of Br is taking place as its oxidation number is decreasing from +5 to -1.
We write the half equations and balance them for everything. Oxygen is balanced by adding
 and hydrogen is balanced by adding
and hydrogen is balanced by adding
 and the charge is balanced by adding electrons.
and the charge is balanced by adding electrons.
Oxidation half reaction:
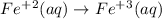
It has equal Fe on both sides and there is no other atom. Only need to balance the charge since it is +2 on left side and +3 on right side. It is balanced by adding one electron to the right side.
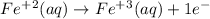
reduction half equation:
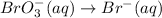
Br is already balanced. Add three water molecules to the right side to balance oxygen.
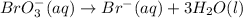
Add six hydrogen ions to the left side to balance hydrogen.
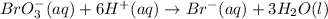
Need to add six electrons to the left side to balance the charge.
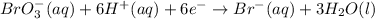
next step is to make the electrons equal and for this we need to multiply the oxidation half-reaction by six.
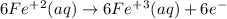
Add the two equations to get the overall equation, If anything is common then cancel this.
The over all equation is:
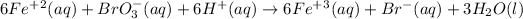
In this equation, the coefficient in front of the
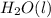 is 3.
is 3.