Answer:
2.068 x 10^6 m / s
Step-by-step explanation:
radius, r = 5.92 x 10^-11 m
mass of electron, m = 9.1 x 10^-31 kg
charge of electron, q = 1.6 x 10^-19 C
As the electron is revolving in a circular path, it experiences a centripetal force which is balanced by the electrostatic force between the electron and the nucleus.
centripetal force =
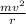
Electrostatic force =
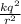
where, k be the Coulombic constant, k = 9 x 10^9 Nm^2 / C^2
So, balancing both the forces we get
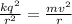
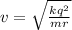
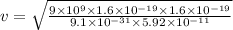
v = 2.068 x 10^6 m / s
Thus, the speed of the electron is give by 2.068 x 10^6 m / s.